

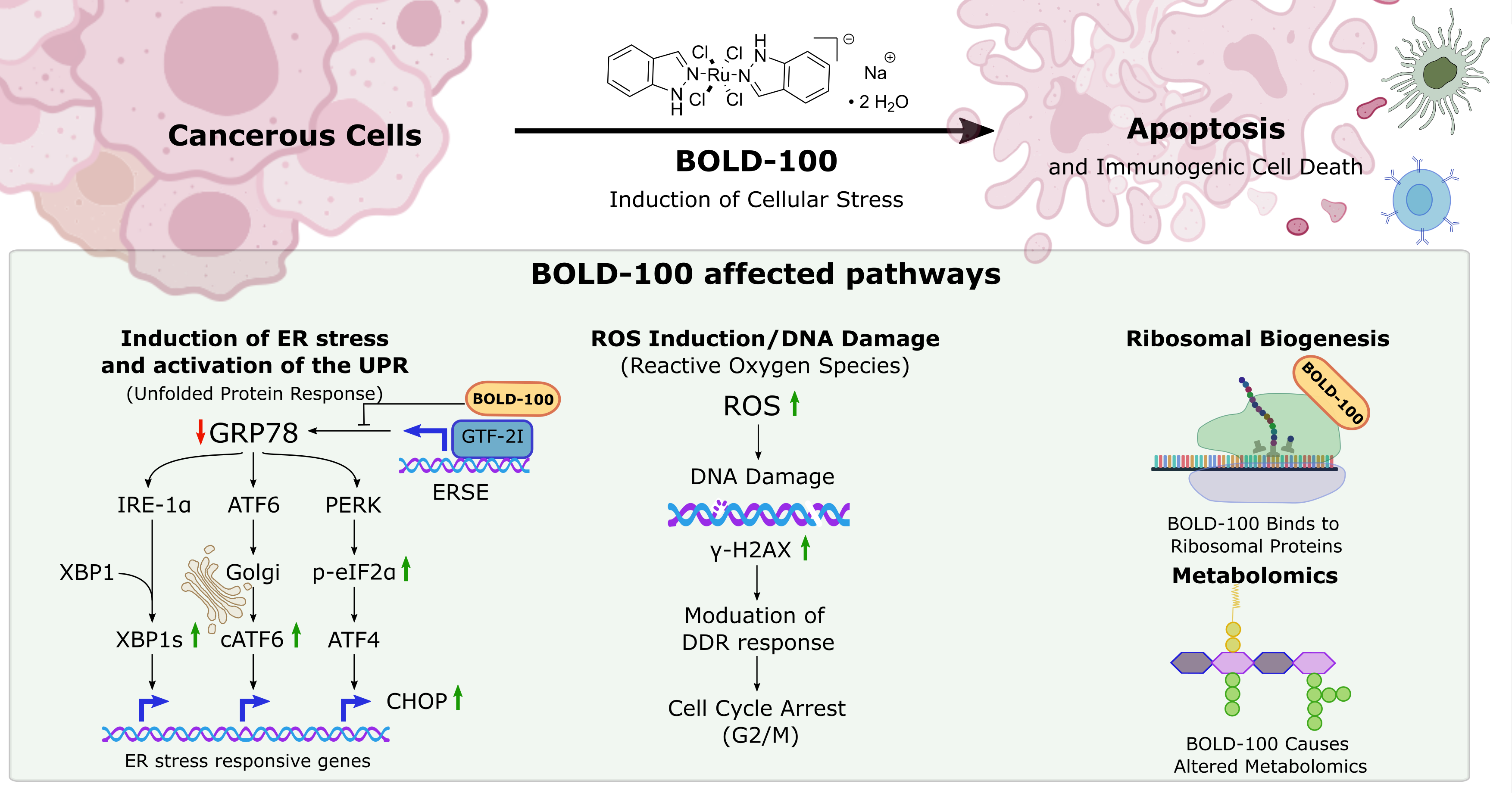
Bold Therapeutics' BOLD-100 is a first-in-class ruthenium-based small molecule therapeutic with a multimodal mechanism-of-action and broad therapeutic potential, high potency, and low systemic toxicity. BOLD-100’s unique mechanism-of-action centers around (1) altering the unfolded protein response (UPR) through selective GRP78 inhibition; and (2) the induction reactive oxygen species (ROS) which causes DNA damage and cell cycle arrest. Collectively, these effects result in cell death in both sensitive and resistant cancers, giving BOLD-100 the potential to significantly improve outcomes in a wide range of both solid and liquid tumors in combination with other anticancer therapies ranging from traditional chemotherapies to targeted therapies to immuno-oncology agents.
Bold Therapeutics is striving to deliver novel therapies for cancer patients in disease areas with significant unmet needs. Together with our network of international collaborators, we are dedicated to the development of our lead product candidate, BOLD-100, a novel metallotherapeutic for the treatment of patients with gastrointestinal cancer. We are committed to improving the lives of patients with these difficult-to-treat cancers.
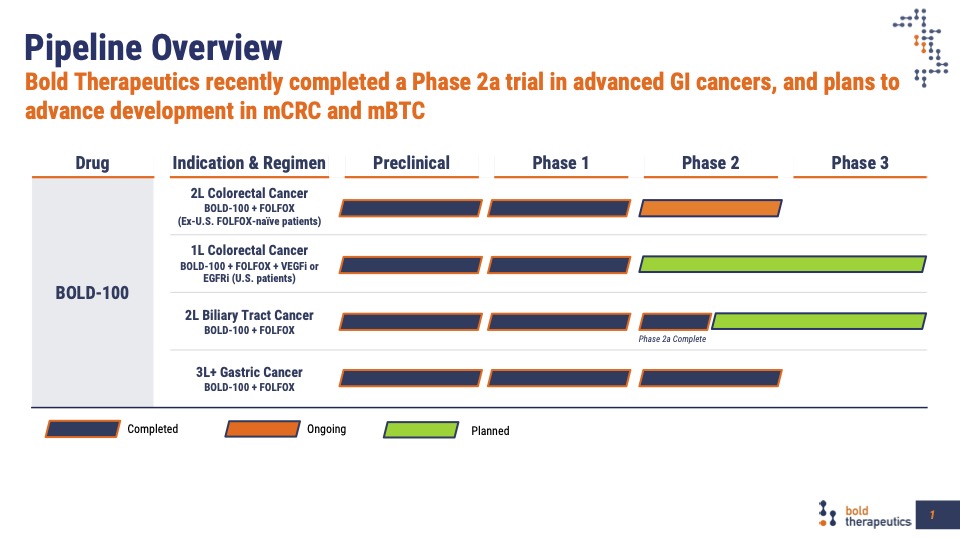
Our clinical development program has treated patients with advanced colon, biliary tract, gastric and pancreatic cancers using a treatment combination of BOLD-100 and FOLFOX. Efficacy data from these studies indicate this treatment results in the improvement of important clinical outcomes including progression-free-survival (PFS), overall survival (OS) and disease control rates. As important, BOLD-100 can be safely combined with FOLFOX therapy, with no new Grade 3 or 4 treatment-emergent adverse events. Patients treated or rechallenged with oxaliplatin can develop oxaliplatin-induced peripheral neuropathy which can be dose and treatment limiting. Preliminary results indicate that fewer than expected number of patients treated with BOLD-100 and FOLFOX developed this adverse event indicating a neuroprotective effect; and patients can safely remain on treatment for an extended number of treatment cycles. Follow-on studies will further define and characterize this important product attribute.
Compelling Phase 2 data in these areas of has been presented at recent medical meetings:
The BOLD-100 development program builds on the Phase 2 efficacy and safety data indicating multiple opportunities for further randomized clinical trials.
Treatment of oxaliplatin naive metastatic colorectal cancer patients in the first or second-line setting with BOLD-100 in combination with FOLFOX standard of care combinations are ongoing and planned. This is a large patient population in need of better therapeutic outcomes.
Patients with advanced metastatic colorectal cancer have few treatment options. In the completed Phase 2 study, patients with advanced disease and treated with BOLD-100 in combination with FOLFOX demonstrated improved progression-free survival, overall survival, and disease control rates compared to historically expected outcomes and no new treatment emergent adverse events. A randomized, controlled clinical trial in this patient population will be conducted.
Biliary cancer patients have limited treatment options with poor outcomes when treated with current standard of care therapy. Preliminary Phase 2 data treating patients with relapsed or refractory disease provides evidence to support further investigation in a planned, randomized study.
Bold Therapeutics is committed to scientific and clinical research to advance and improve outcomes for all patients with cancer. Additional clinical trials will continue to address important scientific questions and support the development of our investigational medicines as we pursue the goal of providing new treatment options to patients with advanced gastrointestinal cancers.
Please visit ClinicalTrials.gov for more information.
Pursuant to Article 27 of the General Data Protection Regulation (GDPR), Bold Therapeutics Inc. has appointed European Data Protection Office (EDPO) as its GDPR Representative in the EU. You can contact EDPO regarding matters pertaining to the GDPR:

BOLD-100 is an anticancer agent being developed in combination with standard-of-care therapies, to improve patient outcomes in difficult to treat cancers. Most current treatment strategies for advanced cancers are either effective in only some patient populations or have high relapse rates. BOLD-100 is designed to be used in combination with these treatments to improve patient outcomes. In preclinical studies, BOLD-100 enhances a wide range of therapies, including standard of care agents like chemotherapies and targeted agents, but also novel drugs currently in development like ATR inhibitors.
BOLD-100 has a unique, multimodal mechanism-of-action that selectively kills cancerous cells. Specifically, BOLD-100 alters the unfolded protein response (UPR) through selective GRP78 inhibition, leading to endoplasmic reticulum stress, activation of the unfolded protein response, and cellular death via apoptosis. Additionally, cancer cells utilize this pathway to become resistant to standard of care therapies - by targeting this pathway, BOLD-100 is able to overcome this resistance, improving outcomes in both sensitive and resistant cancers.
BOLD-100 also induces reactive oxygen species (ROS) which causes DNA damage and cell cycle arrest. DNA damaging agents are a mainstay of cancer therapy - by also activating this pathway, BOLD-100 can potentiate the effect of current cancer therapies, demonstrating the unique potential of combinational strategies with BOLD-100 to potentiate efficacy while overcoming resistance.
Bold Therapeutics is developing a robust pipeline of novel metallotherapeutics. We have developed an innovative, best-in-class screening platform to identify metallotherapeutics with high anticancer activity and low toxicity. Leveraging the unique properties of metallotherapeutics, this screening program is specifically designed to identify compounds with novel anticancer mechanisms. Working with an extensive global network of leading academic chemists, Bold Therapeutic has access to the most innovate metallotherapeutic compounds in development. Research and development activities for pipeline expansion with BOLD-200 and BOLD-300 are ongoing while screening continues in the search for new and interesting metallotherapeutics. We encourage potential collaborators to connect with us here.
Bold Therapeutics is working with a leading team of international partners, clinicians and collaborators to develop BOLD-100. We believe collaborations and partnerships are the key to scientific discovery and advancement. Connect with us if you’re interested in collaborating with us to explore the potential of BOLD-100.
Connect With UsBOLD-100 and its precursor molecules’ (KP1339, NKP-1339, IT-139) clinical scientific and clinical advancements have been presented and published in dozens of peer-reviewed forums.
A full list of publications is available here.
Select papers / posters:
ASCO 2024 Poster: O'Kane, G., Oh, D.-Y., Spratlin, J., Rha, S. Y., Elimova, E., Kavan, P., ... Pankovich, J. (2024, May 31 - June 4). A phase 2 study of BOLD-100 in combination with FOLFOX chemotherapy in patients with pretreated advanced biliary tract cancer: Efficacy and safety analysis [Poster presentation]. American Society of Clinical Oncology (ASCO) Annual Conference, Chicago, IL. View
Spratlin, J., Rha, S. Y., O’Kane, G., Oh, D.-Y., Elimova, E., Goodwin, R., Kim, S. T., Koo, D.-H., McAllister, E. R., Jones, M., Snow, M., Lemmerick, Y., & Spera, G. (2024, May 31 – June 4). A phase 2 study of BOLD-100 in combination with FOLFOX chemotherapy in patients with advanced gastric cancer: Efficacy and safety analysis [BOLD-100-001]. Presented at the American Society of Clinical Oncology (ASCO) Annual Conference, Chicago, IL. Bold Therapeutics, Inc. View
ESMO 2024 Poster: O'Kane, G., Spratlin, J., Oh, D.-Y., Rha, S. Y., McWhirter, E., Elimova, E., ... Pankovich, J. (2024, June 26-29). BOLD-100-001: A phase 2 study of BOLD-100 in combination with FOLFOX in advanced mCRC patients that have failed at least two prior lines of therapy [Poster presentation]. European Society for Medical Oncology (ESMO) Annual Congress for Gastrointestinal Cancers, Munich, Germany and Online. View
Author, A. A., Author, B. B., & Author, C. C. (Year). Ruthenium drug BOLD-100 regulates BRAFMT. Molecular Cancer Research, Volume(Issue), Pages. View
Spratlin JL, O’Kane G, Goodwin RA, et al. BOLD-100-001 (TRIO039): A phase 1b dose-escalation study of BOLD-100 in combination with FOLFOX chemotherapy in patients with advanced gastrointestinal solid cancers: Interim safety, tolerability, and efficacy. ASCO 2022 Abstract. View
Bakewell S, Conde I, Fallah Y, et al. Inhibition of DNA Repair Pathways and Induction of ROS Are Potential Mechanisms of Action of the Small Molecule Inhibitor BOLD-100 in Breast Cancer. Cancers (Basel). 2020 Sep 16;12(9):2647. View
Neuditschko B, Legin AA, Baier D, et al. Interaction with Ribosomal Proteins Accompanies Stress Induction of the Anticancer Metallodrug BOLD-100/KP1339 in the Endoplasmic Reticulum. Angew Chem Int Ed Engl. 2021 Mar 1;60(10):5063-5068. View
Burris HA, Bakewell S, Bendell JC, et al. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open. 2017;1(6):e000154. Published 2017 Feb 23. View
Bakewell SJ, Rangel DF, Ha DP, et al. Suppression of stress induction of the 78-kilodalton glucose regulated protein (GRP78) in cancer by IT-139, an anti-tumor ruthenium small molecule inhibitor. Oncotarget. 2018;9(51):29698–29714. Published 2018 Jul 3. View
Heffeter P, Atil B, Kryeziu K, et al. The ruthenium compound KP1339 potentiates the anticancer activity of sorafenib in vitro and in vivo. Eur J Cancer. 2013;49(15):3366–3375. View
Gifford JB, Huang W, Zeleniak AE, et al. Expression of GRP78, Master Regulator of the Unfolded Protein Response, Increases Chemoresistance in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther. 2016;15(5):1043–1052. View